Abstract
Background: Acalabrutinib is a next-generation, covalent Bruton tyrosine kinase inhibitor approved for the treatment of patients with treatment-naive (TN) or relapsed/refractory chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). A previous analysis of the phase 1/2, multicenter ACE-CL-001 study (NCT02029443) demonstrated durable responses and good long-term tolerability of acalabrutinib as first-line therapy in patients with CLL at a median follow-up of 53 months (Byrd et al. Blood. 2021;137:3327-38). Here, we report the final analysis in the TN cohort of that study, the longest running study of acalabrutinib, with over 6 years of follow-up.
Methods: Patients included in the study were aged ≥18 years with TN CLL/SLL who met the 2008 iwCLL criteria for treatment, were inappropriate for or declined standard chemotherapy, and had ECOG performance status 0-2. All TN patients were treated in the phase 2 portion of the study and received acalabrutinib 100 mg twice daily (BID) or 200 mg once daily (QD), later switching to 100 mg BID, until progressive disease (PD) or unacceptable toxicity. The primary endpoint was safety. Secondary endpoints included investigator-assessed overall response rate (ORR), time to response (TTR), duration of response (DOR), and progression-free survival (PFS), with a post hoc analysis of event-free survival (EFS).
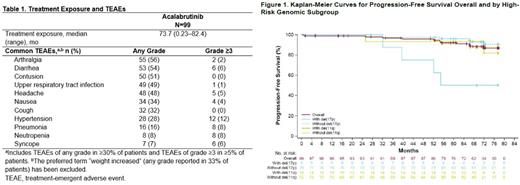
Results: Ninety-nine patients (n=62 100 mg BID; n=37 200 mg QD) were treated. The median age was 64 years; 47% of patients had Rai stage 3-4 disease, 10% had del(17p), 14% had TP53 mutation, 17% had del(11q), 62% had unmutated IGHV, and 18% had complex karyotype. At the final data cut-off date of July 15, 2021, the median study follow-up was 73.7 months (range 0.92-82.4), and 70 (71%) patients remain on acalabrutinib treatment. The most common reasons for treatment discontinuation were adverse events (AEs) (10%) and PD (6%).
The most common AEs (Table 1) were consistent with those reported in the prior update (53-month median follow-up). The only grade ≥3 AEs with a ≥50% increase in incidence since the prior update were pneumonia (4% [prior update] vs 8%) and syncope (4% [prior update] vs 6%). AEs that led to treatment discontinuation were amyotrophic lateral sclerosis, angiosarcoma, cardiac failure, cerebral hemorrhage, gastric hemorrhage, glioblastoma multiforme, non-cardiac chest pain, prostate cancer, sepsis, small cell lung cancer, and urinary tract infection (in 1 patient each). All-grade and grade ≥3 events of clinical interest included infection (87% and 19%, respectively), bleeding events (74%, 7%), major bleeding events (8%, 7%), and hypertension (29%, 13%). Atrial fibrillation/flutter occurred in 6% of patients (3% grade ≥3). Second primary malignancies excluding non-melanoma skin (all grades) occurred in 14% of patients Two deaths were reported (cardiac failure, n=1; multiple organ dysfunction syndrome, n=1); neither was considered treatment related.
ORR was 97% (9% complete response; 88% partial response). Median TTR was 3.7 months (range 1.7-22.1). Median DOR was not reached; the estimated 66-month DOR rate was 89% (95% CI 80-94). ORR of 100% was seen across high-risk groups: del(17p) (9/9), del(11q) (15/15), unmutated IGHV (57/57), and complex karyotype (12/12). Median PFS was not reached; the estimated 72-month PFS rate was 87% (95% CI 77-93; Figure 1). The estimated 72-month EFS was 78% (95% CI 68-85). Reported EFS events overall included AEs (n=10/99, 10%), disease progression (n=9/99, 9%), and new anticancer therapy (n=3/99, 3%).
Conclusions: The final data from ACE-CL-001 further support the long-term efficacy and safety of acalabrutinib monotherapy, with high response rates and rapid, durable responses seen in patients with TN CLL regardless of high-risk genomic features. With over 6 years of follow-up, this study firmly establishes the tolerability and safety profile of acalabrutinib seen consistently in subsequent studies, with a low incidence of atrial fibrillation/flutter seen and no new long-term safety issues identified.
Disclosures
Byrd:AbbVie: Consultancy; Zencor: Research Funding; Pharmacyclics: Research Funding; Kura: Consultancy; Janssen: Consultancy; Novartis: Consultancy; AstraZeneca: Consultancy; Ohio State University: Patents & Royalties; Trillium: Consultancy; Vincerx: Consultancy, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company; Syndax: Consultancy; Newave: Consultancy. Woyach:Newave: Consultancy; Pharmacyclics: Consultancy; Genentech: Consultancy; BeiGene: Consultancy; AbbVie: Consultancy, Research Funding; Schrodinger: Research Funding; ArQule: Consultancy; Karyopharm Therapeutics: Research Funding; MorphoSys: Consultancy, Research Funding; Loxo@Lilly: Research Funding; Janssen: Consultancy; AstraZeneca: Consultancy. Furman:AbbVie, AstraZeneca, Beigene, BMS, Genentech, Janssen, Loxo, MEI Pharma, Pharmacyclics, Sanofi, TG Therapeutics, X4 Pharmaceuticals: Consultancy. Martin:ADCT: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; BMS: Consultancy; Daiichi Sankyo: Consultancy; Epizyme: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Regeneron: Consultancy; Takeda: Consultancy. O'Brien:Acerta, Alliance, Beigene Ltd, Caribou Biosciences Inc, Gilead, Kite, Loxo Oncology, Mustang, Nurix Therapeutics Inc, Pfizer, Pharmacyclics, Regeneron, Sunesis, and TG Therapeutics.: Research Funding; AbbVie, Alexion, Amgen, Aptose Biosciences, Astellas, AstraZeneca, Autolus, Bristol Myers Squibb, Celgene, DynaMed, Eli Lilly and Company, Gilead, GlaxoSmithKline, Janssen Oncology, Johnson and Johnson, Juno Therapeutics, MEI Pharma Inc, Merck, NOVA Resea: Consultancy. Brown:BeiGene, Gilead, Loxo/Lilly, MEI Pharma, SecuraBio, Sun, TG Therapeutics: Research Funding; Abbvie, Acerta/Astra-Zeneca, BeiGene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Eli Lilly, Genentech/Roche, Hutchmed, iOnctura, Janssen, MEI Pharma, Pharmacyclics: Consultancy. Stephens:Novartis: Research Funding; AstraZeneca: Consultancy; AbbVie: Consultancy; Mingsight: Research Funding; Acerta: Research Funding; Arqule: Research Funding; JUNO: Research Funding; Karyopharm: Research Funding; Beigene: Consultancy; CSL Behring: Consultancy; Genentech: Consultancy; Lilly: Consultancy; Newave: Research Funding; Epizyme: Consultancy; TG Therapeutics: Consultancy; Celgene: Consultancy. Barrientos:Pharmcyclics/AbbVie: Consultancy; AstraZeneca: Consultancy, Research Funding; Beigene: Consultancy; Oncternal: Research Funding; VelosBio, Inc.: Research Funding. Patten:Beigene: Honoraria; AstraZeneca: Honoraria; Abbvie: Honoraria; Gilead Sciences: Honoraria, Research Funding; Roche: Research Funding; Janssen: Honoraria. Munir:Janssen, AstraZeneca, Alexion, Sobi, Novartis, Roche, Abbvie, Gilead: Honoraria; Janssen, AstraZeneca, Alexion, Abbvie, Novartis, Roche: Membership on an entity's Board of Directors or advisory committees. Patel:Abbvie, ADC Therapeutics, AstraZeneca, Beigene, BMS, Caribou Biosciences, Celgene, Epizyme, Roche/Gen, Kite, MEI, Morphosys, Pharmacyclics/Janssen, TG, Trillium Therapeutics/Pfizer, Xencor: Consultancy; Adaptive Biotechnologies, AptevoTherapeutics, AstraZeneca, BMS, Celgene, CRISPR, Curis, Epizyme, Fate Therapeutics, Roche/Gen, Kite, MEI, Nurix, Pharmacyclics/Janssen, Sunesis Pharmaceuticals, Trillium Therapeutics/Pfizer, Velos Bio, Xencor: Research Funding; AstraZenca, BMS, Celgene, Roche/Gen, Kite, Pharmacyclics/Janssen, TG: Speakers Bureau. Butturini:AstraZeneca: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. de Borja:Roche: Current equity holder in publicly-traded company; AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Wang:AstraZeneca: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Jain:Cellectis: Honoraria, Research Funding; Medisix: Research Funding; Servier Pharmaceuticals LLC: Research Funding; MEI Pharma: Honoraria; Ipsen: Honoraria; CareDx: Honoraria; Mingsight: Research Funding; Novalgen: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; TG Therapeutics: Honoraria; Beigene: Honoraria; Pfizer: Research Funding; Takeda: Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; Cellectis: Honoraria, Research Funding; ADC Therapeutics: Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Aprea Therapeutics: Research Funding; Loxo Oncology: Research Funding; Dialectic Therapeutics: Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; Fate Therapeutics: Research Funding; TransThera Sciences: Research Funding; Newave: Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; Incyte Corporation: Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding. Wierda:GSK/Novartis: Research Funding; Gilead Sciences: Research Funding; Genentech: Research Funding; Kite, a Gilead Company: Research Funding; Xencor: Research Funding; Bristol Meyers Squibb (Juno and Celgene): Research Funding; Sunesis: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Pharmacyclics LLC: Research Funding; Genzyme: Consultancy; Karyopharm: Research Funding; Janssen: Research Funding; Cyclacel: Research Funding; Miragen: Research Funding; Sanofi: Consultancy; Loxo Oncology, Inc./Lilly: Research Funding; Juno: Research Funding; AstraZeneca/Acerta Pharma. Inc.: Research Funding; AbbVie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal